Multi Format Vials
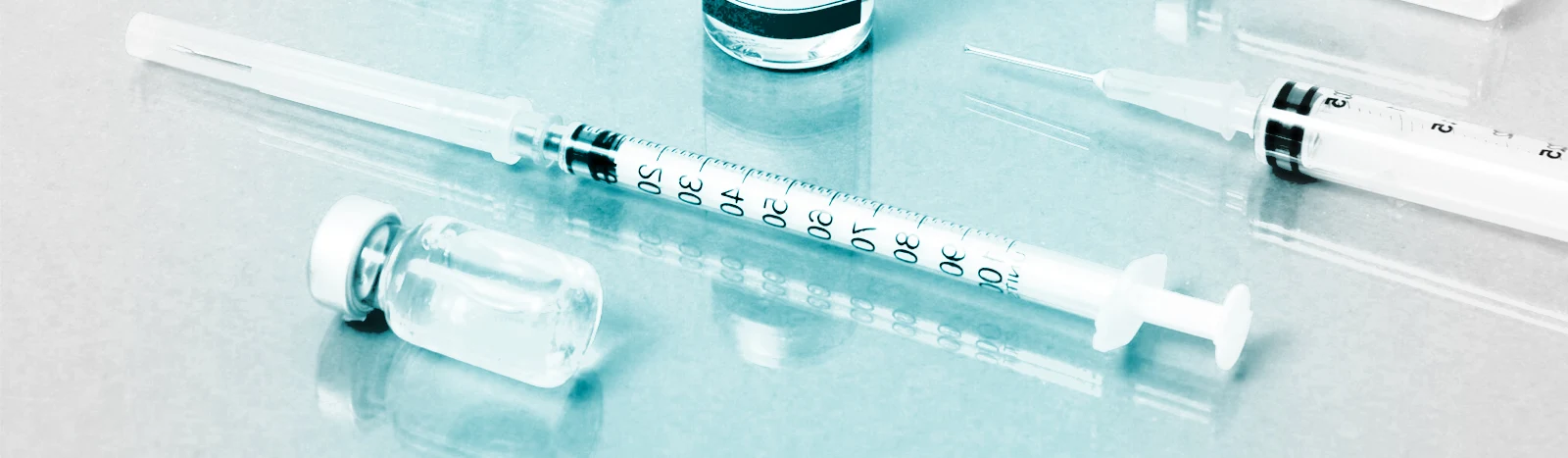
BioCina has extensive capabilities to help differentiate your products in the market through a selection of specialized dosage formats including vials, prefilled syringes, cartridges, bottles, and ampoules. Capabilities include the development, manufacturer, and packaging of a wide variety of products from oral solutions to surface disinfectants, to topical antiseptics. As a leader in plastic pharmaceutical single dose formats, you can be confident in our capabilities to formulate a scalable manufacturing process from small batch clinical trials to multi-format commercial volumes all handled with uncompromised quality.
BioCina offers a diverse group of products and presentations including:
- Aseptic fill vials, syringes, cartridges and ampoules
- Terminally sterilized vials, visual inspection, and vial packaging
- Bottle filling
Multi-format include:
- Wide range of batch sizes from 350L to 4,200L
- Filling of oxygen and metal sensitive products
- Plastic or Glass containers
- 5M+ unit capacity
Comprehensive Services for Your Every Need

Quality &
Regulatory
We produce mRNA drug substance of the highest quality standards, in full compliance with all global regulatory requirements.

Process
Development
BioCina’s expert Poly Nucleic Acid Products team is equipped to develop end-to-end mRNA manufacturing processes up to 1 L IVT and 1 L LNP encapsulation scale.

Analytical
Development
Robust and compliant test methods are developed to ensure the quality, safety and efficacy of all products manufactured at BioCina.

cGMP
Manufacturing
End-to-end cGMP manufacturing of LNP encapsulated mRNA, including cell line development and cGMP manufacture of template plasmid DNA.

Technology
Transfer
Our highly structured approach, including comprehensive gap analysis and robust risk mitigation, ensures reliable technology and process transfers.
Our Expertise On Demand
Thought Leadership & Resources



